Surgical Anatomy - Understanding The Human Body For Surgical Procedures
Surgical anatomy refers to the study of the structure and organization of the human body in relation to surgical procedures. Understanding the anatomy of the body is essential for any surgeon as it helps in the identification and visualization of the different structures, and reduces the risk of complications during surgery.
Author:Suleman ShahReviewer:Han JuApr 07, 202363 Shares930 Views
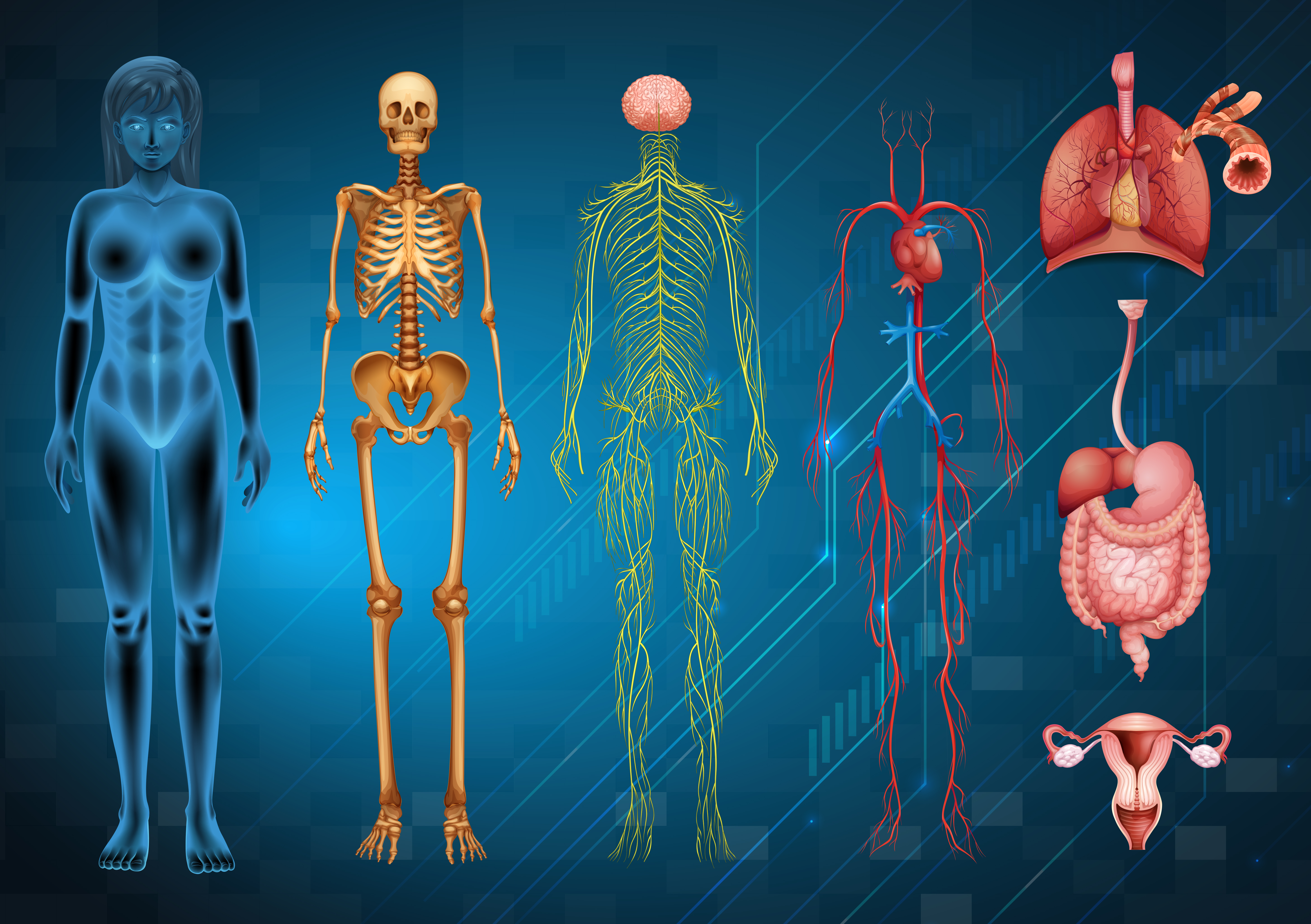
Surgical anatomyrefers to the study of the structure and organization of the human body in relation to surgical procedures.
Understanding the anatomy of the body is essential for any surgeon as it helps in the identification and visualization of the different structures, and reduces the risk of complications during surgery.
In this article, we will discuss the importance of surgical anatomy, its different aspects, and how it can aid in successful surgical outcomes.
What Is Surgical Anatomy?
Surgical anatomy is the study of the anatomy of the human body from the perspective of surgery.
It is a branch of anatomy that focuses on the structures and relationships of the body that are relevant to surgical procedures.
This includes knowledge of the location and function of organs, bones, muscles, nerves, and blood vessels in the body.
Surgical anatomy is essential for surgeons to perform safe and effective surgeries, and it is also used to teach medical students and residents about the anatomy of the human body in a surgical context.
Importance Of Surgical Anatomy
Surgical anatomy is the study of human anatomy as it relates to surgical procedures. It is a critical aspect of surgical education and training, as well as surgical practice.
Here are some of the key reasons why surgical anatomy is important:
Understanding Anatomical Structures
Surgeons need to have a thorough understanding of the anatomy of the human body, including the organs, blood vessels, nerves, and other structures. This knowledge is essential for performing surgical procedures safely and effectively.
Avoiding Complications
By understanding the anatomy of the human body, surgeons can avoid complications during surgery.
For example, they can avoid damaging important structures such as nerves and blood vessels, or they can identify potential problem areas and take steps to prevent complications.
Customizing Procedures
Every patient is different, and surgical anatomy helps surgeons to customize procedures to the specific needs of each patient.
For example, surgeons can use their knowledge of anatomy to select the best surgical approach, or to adapt a procedure to accommodate the patient's unique anatomy.
Improving Outcomes
By understanding the anatomy of the human body and using that knowledge to tailor surgical procedures, surgeons can improve outcomes for their patients. This can lead to better patient satisfaction, faster recovery times, and lower rates of complications.
The Different Aspects Of Surgical Anatomy
Surgical anatomy can be divided into three broad categories:
Regional Anatomy
Regional anatomy involves the study of the structures present in specific regions of the body, such as the abdomen, thorax, and head and neck.
This knowledge helps in identifying the structures that may be affected during surgery and helps in developing surgical plans accordingly.
Surface Anatomy
Surface anatomy is the study of the external features of the body and their relationship with the underlying structures.
This knowledge is essential for identifying the location of vital structures, such as blood vessels and nerves, which are not visible on the surface.
Microscopic Anatomy
Microscopic anatomy involves the study of the structures present at the microscopic level, such as cells, tissues, and organs. This knowledge is essential for understanding the pathophysiology of diseases and the mechanisms of action of different drugs and treatments.
The Role Of Imaging Techniques In Surgical Anatomy
Imaging techniques play a crucial role in surgical anatomy as they provide detailed information about the internal structures of the body.
Different imaging techniques such as X-rays, CT scans, MRI, and ultrasound are used to visualize various body parts and organs before surgical procedures.
X-rays are often used to diagnose bone fractures and dislocations, while CT scans and MRI can provide detailed images of soft tissues such as muscles, tendons, and ligaments. Ultrasound is commonly used to visualize organs such as the heart, liver, and kidneys.
By using these imaging techniques, surgeons can determine the exact location and size of the structures they need to operate on. This helps them plan the surgical approach and reduces the risk of complications during the procedure.
In addition, imaging techniques can be used to monitor the progress of the surgery and assess the success of the procedure.
This is particularly important in complex surgeries where the surgeon needs to navigate through multiple structures and organs to reach the target area.
Overall, the use of imaging techniques in surgical anatomy has revolutionized the field of surgery, allowing for more precise and successful surgeries with fewer complications.
Clinical Applications Of Surgical Anatomy
Surgical anatomy has several clinical applications in the field of medicine. Here are some of them:
1. Surgical Planning And Execution
Surgical anatomy helps in the planning and execution of surgical procedures. Surgeons need to have a thorough understanding of the surgical anatomy of the region where they will operate to avoid injury to vital structures and to ensure a successful outcome.
2. Diagnosis And Treatment Of Diseases
Knowledge of surgical anatomy helps in the diagnosis and treatment of various diseases. For example, a tumor in a specific region of the body will affect the surrounding anatomical structures.
Knowledge of surgical anatomy will help the surgeon to diagnose the disease and plan the best course of treatment.
3. Minimally Invasive Surgery
Minimally invasive surgery requires a thorough understanding of the anatomy of the surgical site to ensure that the procedure is performed safely and effectively.
4. Teaching And Training
Surgical anatomy is an essential component of medical education. Medical students need to have a thorough understanding of surgical anatomy to become competent surgeons.
Surgical anatomy is taught in medical schools and residency programs to provide students with a foundation for their surgical education.
5. Research
Surgical anatomy is an essential component of surgical research. Researchers use anatomical knowledge to develop new surgical procedures, devices, and technologies. The study of surgical anatomy helps to improve surgical outcomes and patient care.
Surgical Anatomy Of The Head And Neck
The head and neck are complex anatomical regions that require a thorough understanding of the surgical anatomy for successful surgeries.
The surgical anatomy of the head and neck involves a wide range of structures, including bones, muscles, nerves, blood vessels, and organs. Here are some key aspects of the surgical anatomy of the head and neck:
Bones
The skull is made up of several bones, including the frontal bone, parietal bones, occipital bone, temporal bones, and sphenoid bone.
The facial bones include the maxilla, mandible, zygomatic bones, nasal bones, lacrimal bones, palatine bones, and inferior nasal conchae.
Muscles
The muscles of the head and neck include the muscles of mastication, which move the jaw during chewing, as well as the muscles that control facial expression, speech, and swallowing.
Nerves
The nerves of the head and neck include the cranial nerves, which emerge from the brain and control functions such as vision, hearing, and facial movement.
Other important nerves in the head and neck include the trigeminal nerve, which provides sensation to the face and controls the muscles of mastication, and the facial nerve, which controls facial expression.
Blood Vessels
The blood vessels of the head and neck include the carotid arteries, which supply blood to the brain, and the jugular veins, which drain blood from the head and neck.
Organs
The head and neck contain several important organs, including the brain, eyes, ears, nose, mouth, and throat.
Understanding the surgical anatomy of the head and neck is essential for a wide range of procedures, including facial reconstructive surgery, head and neck cancer surgery, and neurosurgery.
Surgical techniques in this area require precise knowledge of the location and function of each structure in order to achieve optimal outcomes.
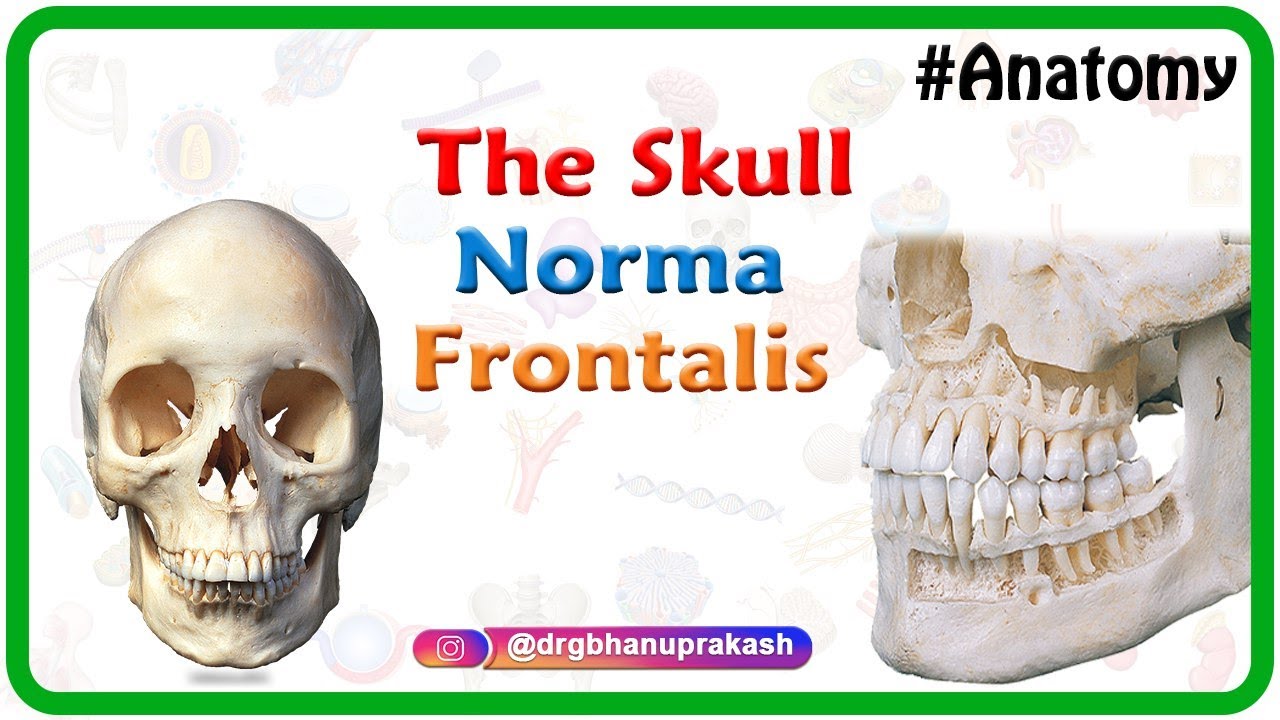
Anatomy of the Skull : Norma Frontalis
People Also Ask
How Is Surgical Anatomy Taught?
Surgical anatomy is typically taught in medical school during anatomy courses, as well as during surgical rotations and training programs. Surgeons also continue to learn and update their knowledge of surgical anatomy throughout their careers.
How Do Surgeons Use Surgical Anatomy In Their Practice?
Surgeons use their knowledge of surgical anatomy to plan surgical procedures, select the appropriate surgical approach, and avoid injuring critical structures. This knowledge also enables them to anticipate potential complications and make necessary adjustments during surgery.
What Are Some Resources For Learning Surgical Anatomy?
Medical textbooks, anatomical atlases, and online resources such as videos and interactive tutorials are all useful resources for learning surgical anatomy. Surgical training programs and continuing education courses can also provide opportunities for surgeons to update their knowledge and skills in surgical anatomy.
Final Thought
Surgical anatomy plays a crucial role in successful surgical outcomes. Surgeons must have a thorough knowledge of the different aspects of surgical anatomy, including regional anatomy, surface anatomy, and microscopic anatomy, to minimize complications during surgery and improve post-operative outcomes.
Advancements in imaging technologyhave further enhanced our understanding of surgical anatomy, making surgical procedures safer and more effective.

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles