Arginine Vasopressin And Pediatric Cardiovascular Surgery [Key Insights]
Arginine vasopressin is a crucial element in pediatric cardiovascular surgery, aiding in recovery and improving patient outcomes.
Author:Suleman ShahReviewer:Han JuOct 21, 202418.1K Shares241.4K Views
Arginine vasopressin has become an important part of the armamentarium available to pediatric cardiac intensive care physicians managing complex infants and children recovering from cardiovascular surgery.
This therapymust be applied cautiously, however, as excessive afterload from its vasoconstrictive effects could be potentially detrimental to this patient population.
Relative arginine vasopressin deficiency has been identified in some children recovering from cardiovascular surgery, and these children likely represent the ideal candidates for arginine vasopressin therapy.
The aim of this critical review is to discuss arginine vasopressin and pediatric cardiovascular surgery.
Preliminary Discussion
As the practice of pediatric cardiovascular surgery has evolved, the armamentarium of medical and technological therapies available to cardiac intensivists has expanded beyond traditional volume resuscitation and catecholamine administration, for example:
- dopamine
- dobutamine
- epinephrine
- norepinephrine
AVP is a peptide hormone consisting of nine amino acids, endogenously produced by the supraoptic and paraventricular nuclei of the hypothalamus and stored in the posterior pituitary gland.
It is released into the systemic circulation in response to numerous stimuli including, but not limited to:
- hyperosmolality
- hypovolemia
- hypoxia
- hypotension
Consequently, AVP has an important role in the maintenance of adequate circulating blood volume and hemodynamic stability, which is achieved through its actions on various end-organ receptors, the predominant of which are:
- systemic vasoconstriction via stimulation of the V1 receptor on vascular smooth muscle
- free water retention via stimulation of V2 receptors on the renal collecting duct
In this review, we aim to discuss what is known in regards to endogenous production and release of AVP in infants and children requiring pediatric cardiac surgery.
Based on these data and reports of exogenous AVP use in this patient population, we aim to define the role of AVP in their post-operative management.
Endogenous Arginine Vasopressin
Plasma AVP concentrations in healthy ‘normal’ children have been reported to be 1 to 7 picograms per milliliter (pg/mL).
Price and colleagues noted significantly elevated plasma AVP concentrations in children with unrepaired left-to-right shunts, 13.9 ± 17.3 pg/mL, as compared with controls, 3.5 ± 1.3 pg/mL, presumably secondary to the congestive heart failureand less than optimal end-organ perfusion often present in these patients.
Several other studies have measured plasma AVP concentrations in children with congenital heart disease in their unrepaired state prior to surgical correction and at multiple time points post-operatively.
Based on these data, it can be concluded that:
- mean plasma AVP concentrations are elevated pre-operatively
- increase further in the immediate post-operative period
- return to baseline values or below by 24 to 48 hours
For many infants and children recovering from cardiovascular surgery, the first 24 to 48 post-operative hours are the most critical, a dynamic state with minute-to-minute changes in circulating blood volume and hemodynamic stability.
The following are all likely to contribute to the increases in endogenous plasma AVP concentrations noted above:
- post-operative low cardiac output
- dysrhythmias
- third-spacing
- (in some cases) hemorrhage
Under these physiologic conditions, administration of additional AVP for therapeutic purposes is not intuitive.
AVP is a potent systemic vasoconstrictor that, when provided in excess, could theoretically worsen cardiac output by adversely affecting loading conditions.
Despite this apparent contradiction, many authors have reported successful use of exogenous AVP therapy to improve hemodynamic stability and end-organ perfusion in this patient population.
See Also: Understanding The Connection Between Nutrition And Cardiovascular Health - A Comprehensive Review
Exogenous Arginine Vasopressin Therapy
The authors of a study published in 1999 by the scientific journal Circulation, with Erika Berman Rosenzweig as lead author, were the first to report the use of AVP in children recovering from cardiac surgery.
In their observational case series, 11 children received exogenous AVP for severe post-operative vasodilatory shock refractory to traditional catecholamines therapy.
Since the predominant hemodynamic effect of AVP is vasoconstriction, patients with vasodilatory shock should improve with AVP. Indeed, in all 11 patients, systolic blood pressure increased following the initiation of therapy and catecholamine dosages were reduced in 10 patients.
The next report of AVP use in this patient population was not published until eight years later.
In a paper published in 2007 by the European Journal of Pediatrics, with Evelyn Lechner as lead author, a series of 17 neonates received exogenous AVP therapy for ‘vasopressor-resistant hypotension’ following cardiopulmonary bypass.
Both systolic and diastolic pressure increased while exogenous catecholamine dosages decreased in all patients.
A paper published in 2008 by the journal Pediatric Critical Care Medicine, with Christopher W. Mastropietro, M.D., as lead author, discussed the impact of AVP to three infants with profound hypoxemia following surgery for complicated single cardiac ventricle anatomy.
According to Dr. Mastropietro and his co-authors, AVP was shown to:
- increase blood pressure
- decrease catecholamine requirements
- improve oxygenation
In this report, the authors utilized the vasoconstrictive action of AVP to increase systemic vascular resistance and encourage pulmonary blood flow in the setting of single ventricle physiology, somewhat analogous to the use of phenylephrine to manage a hypoxemic patient with Tetralogy of Fallot (a type of congenital heart disorder).
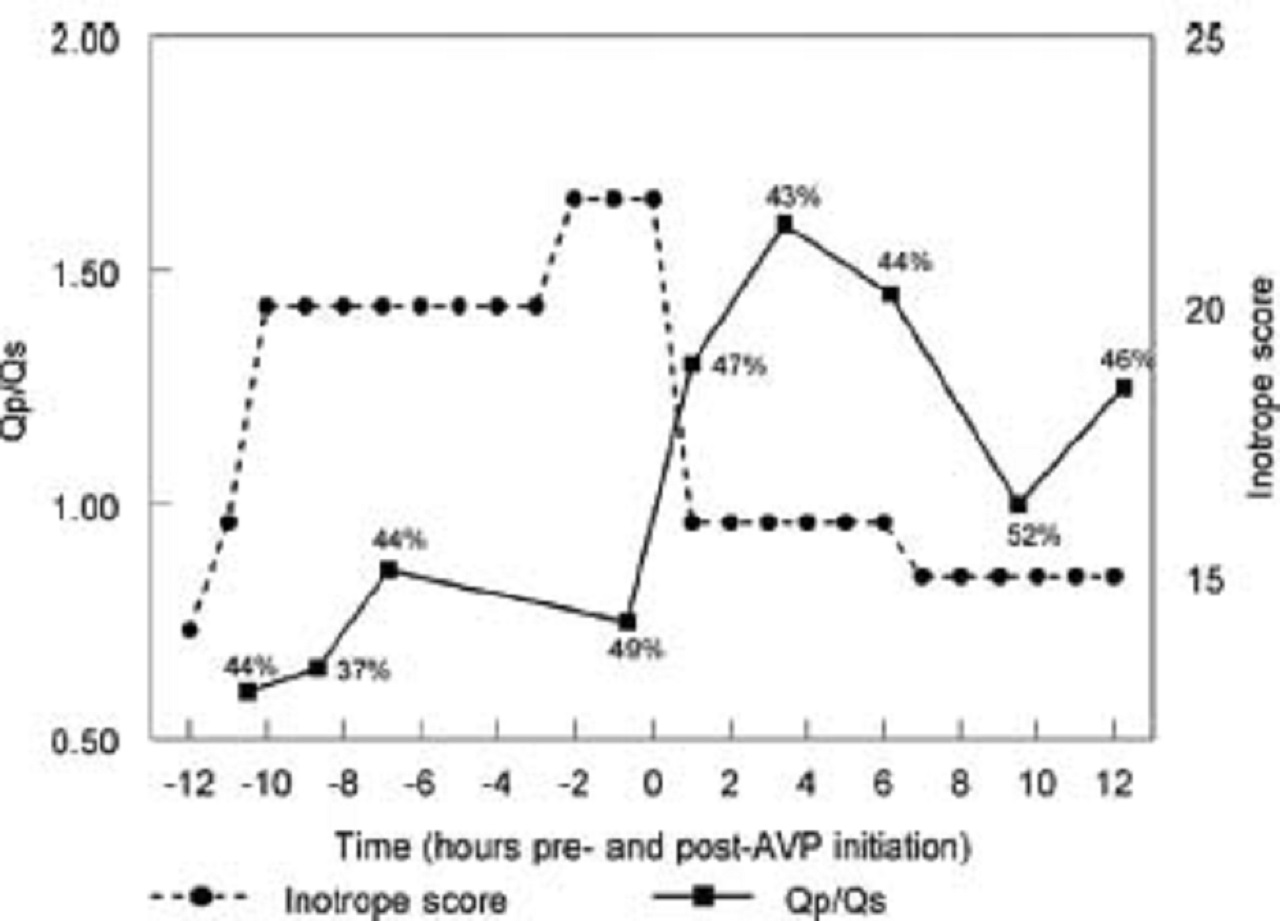
An example of one of these patients is provided in the picture below:
The picture shows the change in pulmonary-to-systemic flow (Qp/Qs) and inotrope score in a patient with hypoxemia following stage I Norwood palliation for hypoplastic left heart disease. After arginine vasopressin initiation, Qp/Qsincreases markedly and remains increased despite weaning the patient’s epinephrine infusion. Measured systemic venous saturation is provided at each time point, which is not adversely affected by AVP therapy.
Moreover, AVP has been shown to decrease the ratio of pulmonary-to-systemic vascular resistance and may decrease pulmonary vascular resistance directly, offering clinicians faced with these patients a theoretical advantage over the more traditional adrenergic vasoconstrictors.
Following these initial encouraging studies, a study published in 2008 by the journal Intensive Care Medicine, the authors, with Nameet Jerath as lead author, described their experience with AVP in a larger cohort of 85 children recovering from cardiac surgery.
Mean catecholamine usage following AVP initiation significantly decreased in these children, but, in contrast to the earlier reports, mean systolic blood pressure was not significantly different from baseline.
It is probable that the earlier smaller observational studies focused solely on those patients in whom AVP use appeared beneficial, whereas this larger report was more inclusive with patients who experienced variable systolic blood pressure changes, likely increasing in some patients while remaining unchanged or even decreasing in others.
This variable response has been reported in other series.
A study published in 2013 by the journal Cardiology in the Young, with Christopher W. Mastropietro, M.D., as lead author, closely examined this variable response in 34 patients with hemodynamic instability after cardiovascular surgery.
In this series, patients were labeled as favorable responders to exogenous AVP therapy if systolic blood pressure increased and exogenous catecholamine requirements decreased after AVP initiation.
Based on this definition, 17 of 34 patients responded favorably to exogenous AVP therapy.
Changes in blood pressure and catecholamine requirements in these patients are illustrated in the picture below:
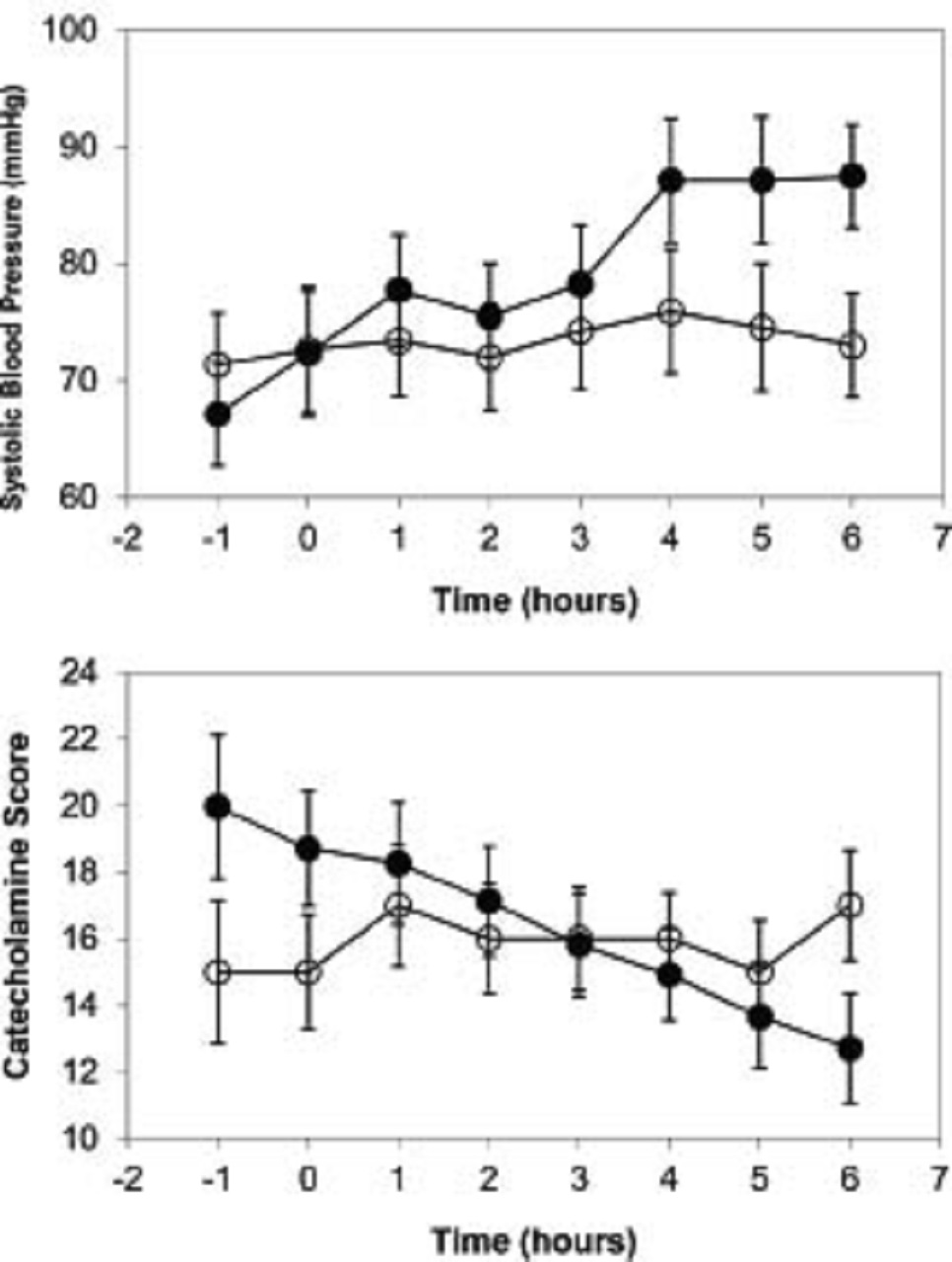
The picture shows the mean hourly changes in (a) systolic blood pressure (SBP) and (b) catecholamine score after initiation of arginine vasopressin (AVP). In patients who responded favorably (dark circles), mean SBP steadily increased and mean catecholamine score steadily decreased after AVP was started (time: 0) while mean SBP and catecholamine score in patients who did not respond or responded unfavorably (white circles) changed minimally. Error bars represent standard error of the mean.
Interestingly, the patients who responded favorably were started on AVP therapy significantly later in the post-operative period, at a median post-operative time of 20 hours (intra-quartile range or IQR: 16 to 27 hours), as compared to 6 hours (IQR: 5.5 to 21.5 hours) in those who did not respond, P=0.032.
These findings are not surprising, considering endogenous plasma AVP concentrations would be expected to be higher 6 hours post-operatively as compared to 20 hours post-operatively.
In other words, exogenous AVP administration would likely be less effective in patients who already have high endogenous plasma AVP concentrations.
Further, hemodynamic compromise on the first post-operative night in children recovering from cardiac surgery is more often due to low cardiac output with elevated systemic vascular resistance rather than systemic vasodilation/vasodilatory shock.
Invasive bedside measurement of systemic vascular resistance following pediatric cardiac surgery is rare, and physical exam as a means to determine systemic vascular resistance is unreliable.
If AVP is administered in patients with low cardiac output and elevated systemic vascular resistance, it would likely be of little benefit and potentially harmful.
Catecholamine requirements did increase in some of the patients who did not respond favorably to AVP, though it is not clear if this clinical worsening was related to AVP therapy.
Relative Deficiency Of Arginine Vasopressin
In the aforementioned first report of exogenous AVP therapy in children after pediatric cardiac surgery, Rosenzweig and colleagues measured plasma AVP concentrations in three of the 11 patients prior to AVP initiation.
The measured AVP concentrations in these patients were:
- 1.9 pg/mL
- 4.4 pg/mL
- 52.4 pg/mL
The authors concluded that these patients, especially the two patients with AVP concentrations in the normal range, had low plasma AVP concentrations relative to what would have been expected given the degree of hypotension.
From these data, they hypothesized the existence of relative AVP deficiency in some children following cardiopulmonary bypass.
This hypothesis was proven correct in a study published in 2010 by the journal Critical Care Medicine, with Christopher W. Mastropietro, M.D., as lead author. The study measured plasma AVP concentrations in 120 pediatric patients before cardiopulmonary bypass and at 4, 24, and 48 hours post-cardiopulmonary bypass.
Dr. Mastropietro and his co-authors a priori (Latin for “from what is earlier”) divided this cohort into two groups:
a. patients with plasma AVP concentrations above the 25th percentile of all values obtained at this time point
b. patients with plasma AVP concentrations below the 25th percentile
The authors hypothesized that plasma AVP concentrations would not be increased in some patients at 4 hours post-cardiopulmonary bypass, and these patients would fall below the 25th percentile of all values measured at this time point.
The results are provided in the picture below:
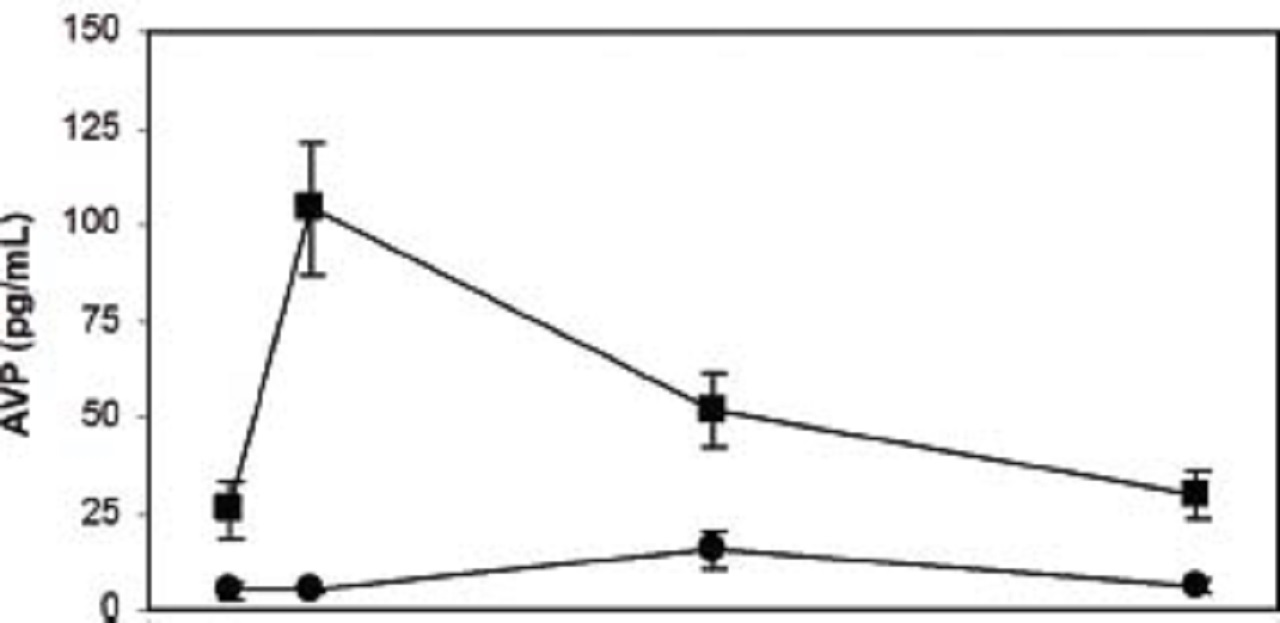
The picture shows the mean ± standard error of plasma arginine vasopressin (AVP) concentration over time in patients with plasma AVP below 25th percentile at 4 hours post-cardiopulmonary bypass (CBP) (circles) as compared to the rest of the population (squares). In patients with AVP concentrations below the 25th percentile (n=29), mean plasma AVP concentration was not increased from baseline at 4, 24, or 48 hours post-CPB. In contrast, mean plasma AVP concentrations in the rest of the cohort (n=90) increased significantly from its baseline at 4 hours post-CPB. Mean plasma AVP concentrations were also significantly lower in the former group pre-CPB and 24 and 48 hours post-CPB. Hemodynamic status (e.g., systemic and central venous pressures, inotrope requirements) and serum sodium did not differ between groups at any time point.
The 25th percentile was determined to be a plasma AVP concentration of 9.2 picograms per deciliter (pg/dL).
In patients with plasma AVP concentrations below this value, mean plasma AVP concentration was within the normal range at baseline (i.e., 1 to 7 pg/mL) and was not significantly increased from this baseline at 4, 24, or 48 hours after cardiopulmonary bypass.
In contrast, mean plasma AVP in the rest of the patients was similar to that which was described previously, which is higher than the normal range at baseline, significantly increased from this baseline at 4 hours after cardiopulmonary bypass, and back to baseline by 24 to 48 hours.
In fact, mean plasma AVP concentrations in the group of patients below the 25th percentile at 4 hours was significantly lower than the rest of the patients at all of time points, including baseline, suggesting that these patients may have an inherent inability to generate an appropriate AVP response.
Established stimuli for endogenous AVP release did not differ between groups at any time point. Such stimuli include changes in:
- blood pressure
- central venous pressure
- serum sodium (i.e., osmolality)
The following were also not statistically different between groups:
- catecholamine requirements
- arterial blood gas measurements
- volume resuscitation
In other words, patients with plasma AVP concentrations below the 25th percentile at 4 hours post-cardiopulmonary bypass were not considered to be “less sick” than the rest of the patients.
Dr. Mastropietro and his co-authors concluded that these patients had “relative AVP deficiency.’’
Though arbitrary, the a priori decision by the authors to use the 25th percentile at 4 hours post-cardiopulmonary bypass was physiologically justified by the results.
It is important to note that many patients with relative AVP deficiency in the aforementioned 2010 study by Mastropietro et al. were not hemodynamically unstable. Hence, relative AVP deficiency in and of itself did not cause hypotension.
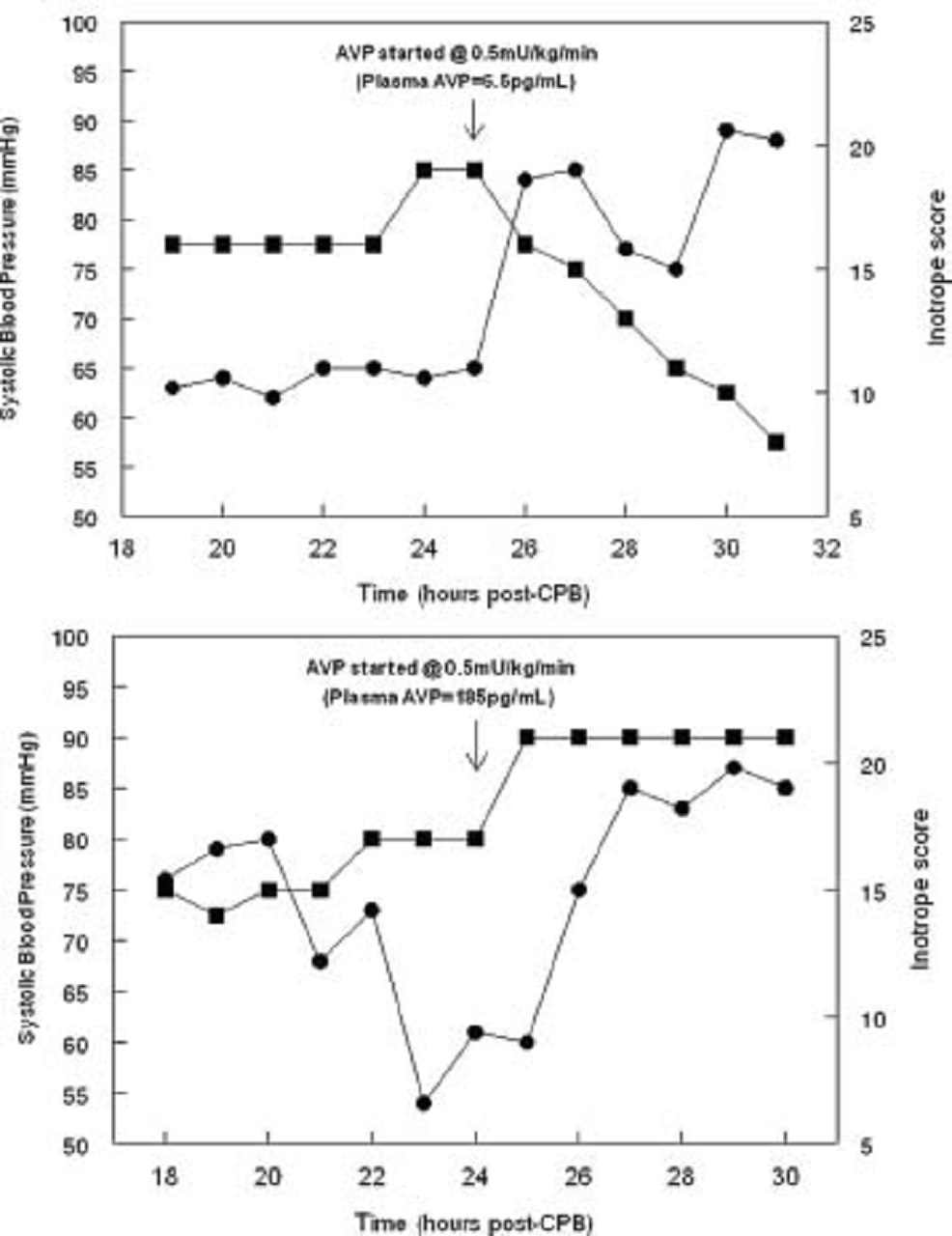
On the other hand, post-operative exogenous AVP infusions deemed necessary by the primary care team to manage hemodynamic instability were initiated in a small subset of patients in this study, and pre-infusion plasma AVP measurements in these patients revealed the importance of identifying those with relative AVP deficiency.
Three of these patients had clinical evidence of hemodynamic instability (e.g., high inotrope requirements) and concomitant low pre-infusion plasma AVP concentrations (< 9.2 pg/mL).
For these children, rapid hemodynamic improvement occurred following the initiation of low-dose exogenous AVP therapy. Within 6 hours, blood pressure increased and catecholamine requirements decreased.
In contrast, two patients with similar degrees of hemodynamic instability had markedly elevated plasma AVP concentrations (175 and 181 pg/mL), and in these children, hemodynamic stability worsened with AVP therapy.
The picture above shows the changes in systolic blood pressure (SBP, circles) and inotrope requirements (squares) of two representative patients after administration of exogenous arginine vasopressin (AVP) on post-operative day 1. (A) Immediately prior to AVP administration, plasma AVP was only 9.1pg/mL despite an inotrope score of 19 and SBP of 64 mmHg. Upon administration, SBP increased to 84 mmHg and remained increased while decreasing catecholamine dosage by >50%. (B) Plasma AVP was 185.1 pg/mL prior to AVP administration in this patient. Not surprisingly, SBP did not increase with exogenous AVP; rather, further inotropic escalation was required.
Therefore, though relative AVP deficiency in and of itself does not cause hypotension, patients with relative AVP deficiency are likely the best candidates for exogenous AVP therapy should hypotension arise.
These data also provided evidence for the notion that hemodynamic response to AVP, at least in part, is dependent on pre-infusion endogenous AVP concentrations.
Unfortunately, relative AVP deficiency in the 2010 study by Mastropietro et al. could not be predicted on the basis of any demographic, anthropometric, or clinical parameters including peripheral skin temperatures, which are used by some as a crude clinical marker of systemic vascular resistance.
Moreover, direct measurement of plasma concentrations is too cumbersome and time-consuming (i.e., at least 48 hours to complete) to provide any value to bedside physicians caring for unstable cardiac patients.
Consequently, we have no current means of practically identifying which of these infants and children have relative AVP deficiency.
For this reason, indiscriminate use of exogenous AVP therapy (i.e., use in patients with elevated endogenous AVP concentrations and/or elevated systemic vascular resistance), at least for the time being, will likely continue to occur.
Copeptin
Copeptin is a byproduct of the AVP precursor pro-vasopressin with an undefined biological function. It is released with AVP in an equimolar ratio, is more stable and much easier to measure.
Plasma copeptin concentrations have been shown to positively correlate with plasma AVP concentrations in adult patients in a variety of clinical scenarios, including post-cardiac surgery.
More recently, a significant positive association was demonstrated between plasma AVP and copeptin concentrations in children undergoing pediatric cardiac surgery.
In this study, plasma AVP and copeptin concentrations were measured at baseline, 4 and 24 hours following cardiopulmonary bypass in 41 children.
The relationship between the values obtained for the 4-hour time point is illustrated in picture below:
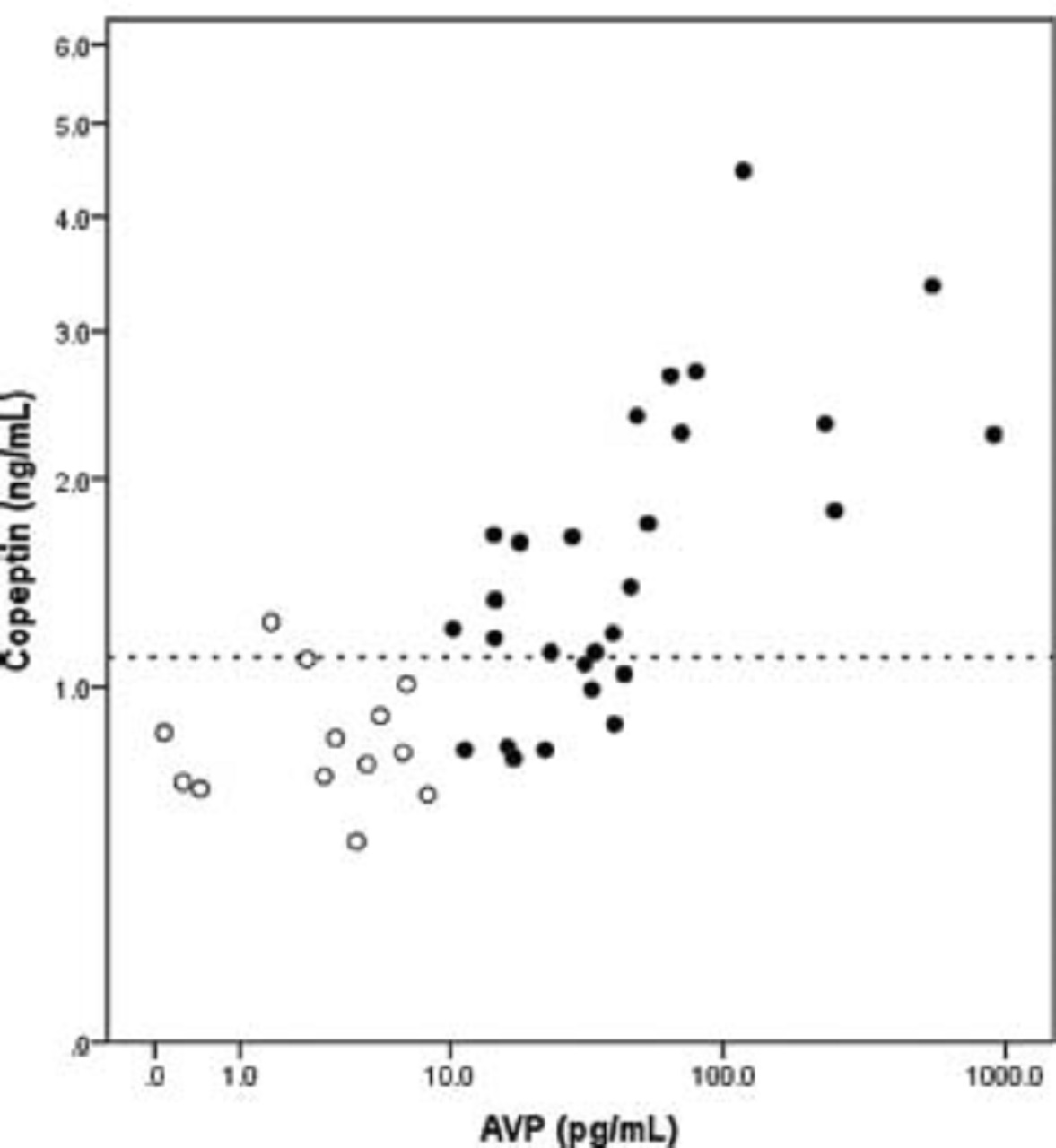
The picture shows plasma arginine vasopressin (AVP) and copeptin measurements obtained at 4 hours post-cardiopulmonary bypass. Both axes were log transformed due to the wide range of plasma AVP concentrations. Patients with relative AVP deficiency are represented by open circles. Dotted Y-axis reference line corresponds to plasma copeptin concentration = 1.12 nanograms per milliliter (ng/mL).
Plasma copeptin concentration less than 1.12 ng/ml at 4 hours post-cardiopulmonary bypass had a sensitivity of 92% (95% confidence intervals: 62 to 100%) and specificity of 71% (95% confidence intervals: 51 to 86%) for relative AVP deficiency (<9.2 pg/mL, as defined in the 2010 study by Mastropietro et al. discussed above), with a negative predictive value of 95% (95% confidence intervals: 74 to 100%).
Using all values obtained from all patients at all three time points, a 1% increase in AVP was associated with a 0.19% increase in copeptin.
Copeptin clearly has potential to be a more easily measured marker of plasma AVP activity and hopefully help identify patients with relative AVP deficiency.
The copeptin assay used in the aforementioned study however requires approximately 6 hours to complete and did not appear to be sensitive enough to accurately measure very low copeptin concentrations.
A different assay, the assay that has been used in most of the previously adult published studies of copeptin, has a lower level of detection and is much faster, delivering the results in less than 45 minutes (Nils Morgenthaler, Institute for Experimental Endocrinology, Berlin, Germany, written communication 26 March 2012).
A larger study including a greater number of patients using this assay should lead to a more precise copeptin definition of relative AVP deficiency.
If AVP and copeptin concentrations can be obtained in some of these patients prior to initiation of an exogenous AVP infusion, a copeptin threshold above which further exogenous AVP therapy is not helpful could be identified.
Conclusion
For the majority of infants and children recovering from cardiovascular surgery, endogenous AVP concentrations will be elevated, especially in the immediate post-operative period, and thus, further AVP provided exogenously will likely be of little benefit and theoretically detrimental.
Judicious use of AVP therapy is therefore warranted.
Based on what we know of AVP pharmacology and the experiences with exogenous AVP in this patient population, children with clinical evidence of systemic vasodilation after the first post-operative night or systemic hypotension in the setting of pulmonary hypertension represent reasonable candidates for AVP therapy.
When initiated, hemodynamic response following AVP initiation should be closely monitored and clinicians should consider discontinuing AVP if clinical improvement does not occur.
Future research focusing on identification of those patients with relative AVP deficiency will hopefully provide a better means of identifying optimal candidates for AVP therapy.
Therefore, more studies about arginine vasopressin and pediatric cardiovascular surgery are highly recommended.

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles